Indian Journal of Pharmacy and Pharmacology
Indian Journal of Pharmacy and Pharmacology (IJPP) is an open-access, peer-reviewed pharmacy journal, published quarterly, as print and online by the Innovative Education and Scientific Research Foundation (IESRF) since 2014. IESRF is dedicated to the transfer of technology and research by publishing scientific journals, research content, providing professional membership, and conducting conferences, seminars, and award programs. With the aim of faster and better dissemination of knowledge, we will be publishing articles ‘Ahe...

Gelucire: A wonderful excipient for improving the physicochemical characteristics of drugs and controlled release matrices
Abstract
The Gelucire family of vehicles is made from a combination of PEG esters of fatty acids and mono-, di-, and triglycerides. These come in a variety of qualities based on their melting point range (33–65 °C) and HLB. They can be used in a wide range of topical and oral preparations. Enhancement of solubility and bioavailability, sustained drug release, taste masking, and protection of active pharmaceutical ingredients (API) against oxygen, light, and humidity are some of the uses for oral formulation. Topical formulations can be used as thickeners, stabilize creams, lotions, and gels, and improve drug absorption via the skin. Fast-release formulations are often prepared using gelucire that contains solely PEG esters. Sustained release formulations are prepared using gelucire that contains either glycerides alone or a combination of glycerides and PEG esters. Their low density and great hydrophobicity make them suitable carriers for the construction of sustained release medication delivery systems.
Introduction
Gelucires are a group of lipid-based excipients made up of glycerides and polyethylene glycol esters, which impart both hydrophobic and hydrophilic characteristics to the formulation. Gelucire is utilized to improve the physicochemical properties of drugs and serve as a matrix for controlled release formulations. Here are some important applications of Gelucire in drug delivery designing.
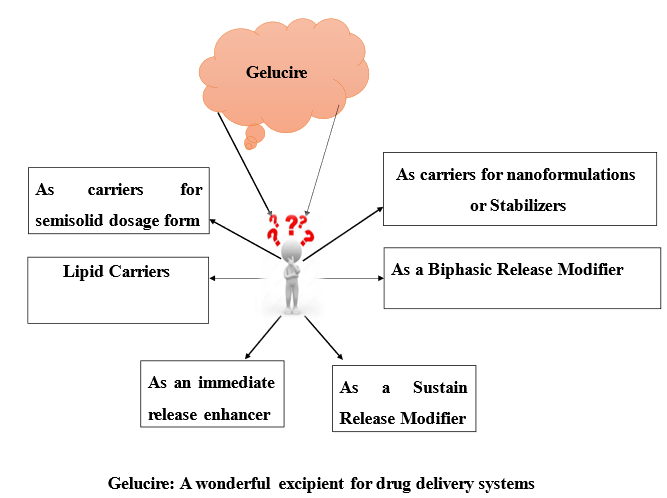
Applications
As lipid carriers
Gelucire is the most used lipid carrier for the preparation of SDD of poorly water-soluble drugs. Gelucire grades differ based on their HLB value, ranging from 1 to 18, and melting point, 33–70 °C. HLB values govern the mechanism of drug release. The fundamental characteristics of Gelucireconsist of the amphiphilic self-assembly characteristics, which means it helps to form fine dispersion of the lipid moiety in the presence of aqueous media.[1]
As an immediate release enhancer
Different grades of monolithic Glucire are used in immediate release formulations and while applying granulation, spray drying, or melt granulation techniques with an optimized amount of disintegrating agent can promote almost complete release of the drug within a few minutes.[2], [3]
As a sustain release modifier
Gelucire containing glycerides or a mixture of glycerides and PEG esters are extremely hydrophobic and very low in density; thus, they are appropriate for sustaining the release of the drug from its delivery systems.[4]
As a biphasic release modifier
Modification of the release profile by means of biphasic delivery can be achieved by using two different grades of gelucire. PEG ester containing Gelucire (50/13) is a rapidly dissolving excipient with hydrophilic nature, whereas only glycerides or mixtures of glycerides and PEG esters containing Gelucire (43/01) are highly hydrophobic and suitable for sustained release preparation. The combination together can provide a loading dose (by immediate release) and maintenance dose (by sustained release) in the form of solid oral dosage of the drug molecules.[5]
As carriers for semisolid dosage form
The Gelucire group of excipients is also widely applied in semi-solid surfactants and matrix formers, which helps in solubility and bioavailability enhancement in lipid-based formulations. Gelucire 48/16 (water-soluble) and Gelucire 44/14 and 50/13 (water-dispersible) variants are mostly used in the semisolid preparation, which shows distinct melting points and HLB values suited for diverse pharmaceutical applications.[6]
As carriers for nano formulations
Gelucire 44/14, with self-emulsifying properties, is used as a nanocarrier system to enhance drug solubility, bioavailability, and stability of the self-nano-emulsification system. Its thermoplasticity supports applications in melt-based drug delivery techniques.[7]
As a stabilizer
Gelucire 50/13 serves as an effective stabilizer for lipid nanocarriers like solid-lipid nanoparticles and nanostructured lipid carriers, enhancing drug loading for hydrophobic drugs. Its bioavailability-boosting properties and compatibility with nanoscale formulations provide significant therapeutic advantages.[8], [9]
Conclusions
Gelucire is used in advanced drug delivery design like in 3D printing technology, demonstrating its versatility as a lipid-based excipient, enabling the fabrication of complex drug delivery systems. The thixotropic properties of gelucire and its compatibility with pharmaceutical semi-solid extrusion and pressure-assisted microsyringe techniques facilitate the development of customizable, patient-specific formulations with enhanced drug solubility and controlled release profiles. [9]
Conflict of Interest
None.
References
- Fernandez S, Rodier J, Ritter N, Mahler B, Demarne F, Carrière F. Lipolysis of the semi-solid self-emulsifying excipient Gelucire44/14 by digestive lipases. . Biochim Biophysica Acta (BBA) Mol Cell Biol Lipids. 2008;1781(6-7):367-75. [Google Scholar]
- Patel D, Patel M, Patel A, Patel C. Formulation and evaluation of mixed matrix gastro-retentive drug delivery for famotidine. Int J Pharm Invest. 2011;1(4):247-54. [Google Scholar]
- Antunes A, Geest B, Vervaet C, Remon J. Gelucire 44/14 based immediate release formulations for poorly water-soluble drugs. Drug Deve Indus Pharm. 2013;39(5):791-8. [Google Scholar]
- Panigrahi K, Patra C, Jena G, Ghose D, Jena J, Panda S. Gelucire: A versatile polymer for modified release drug delivery system. Future J Pharm Sci. 2018;4(1):102-8. [Google Scholar]
- Jammula S, Patra C, Swain S, Panigrahi K, Nayak S, Dinda S. Design and characterization of cefuroxime axetil biphasic floating minitablets. Drug Delivery. 2014;22(1):125-35. [Google Scholar]
- Panda M, Rao M, Panda J, Patra C, Patro G. Gelucire: A flexible formulation excipient. Res J Pharm Technol. 2023;16(2):955-61. [Google Scholar]
- Ghadi R, Dand N. BCS class IV drugs: Highly notorious candidates for formulation development. J Controlled Rel. 2017;248:71-95. [Google Scholar]
- Date A, Vador N, Jagtap A, Nagarsenker M. Lipid nanocarriers (GeluPearl) containing amphiphilic lipid Gelucire 50/13 as a novel stabilizer: Fabrication, characterization, and evaluation for oral drug delivery. Nanotechnology. 2011;22(27). [Google Scholar]
- Ahmad J, Garg A, Mustafa G, Mohammed A, Ahmad M. 3D printing technology as a promising tool to design nanomedicine-based solid dosage forms: Contemporary research and future scope. Pharmaceutics. 2023;15(5). [Google Scholar]
Article Metrics
- Visibility 1k Views
- Downloads 274 Views
- DOI 10.18231/j.ijpp.2024.030
-
CrossMark
- Citation
- Received Date December 11, 2024
- Accepted Date December 14, 2024
- Publication Date December 28, 2024